Read more from Luke
- All Posts
All Posts
- All Posts
CherryCircle Software Makes Austin Chamber’s 2019 Austin A-List for Business Innovation
Luke Guerrero
May 28, 2019
CherryCircle Software, the company providing the QbDVision® platform to accelerate the development of manufacturing processes for the pharmaceutical industry, announced today that the Greater Austin ...
Read more
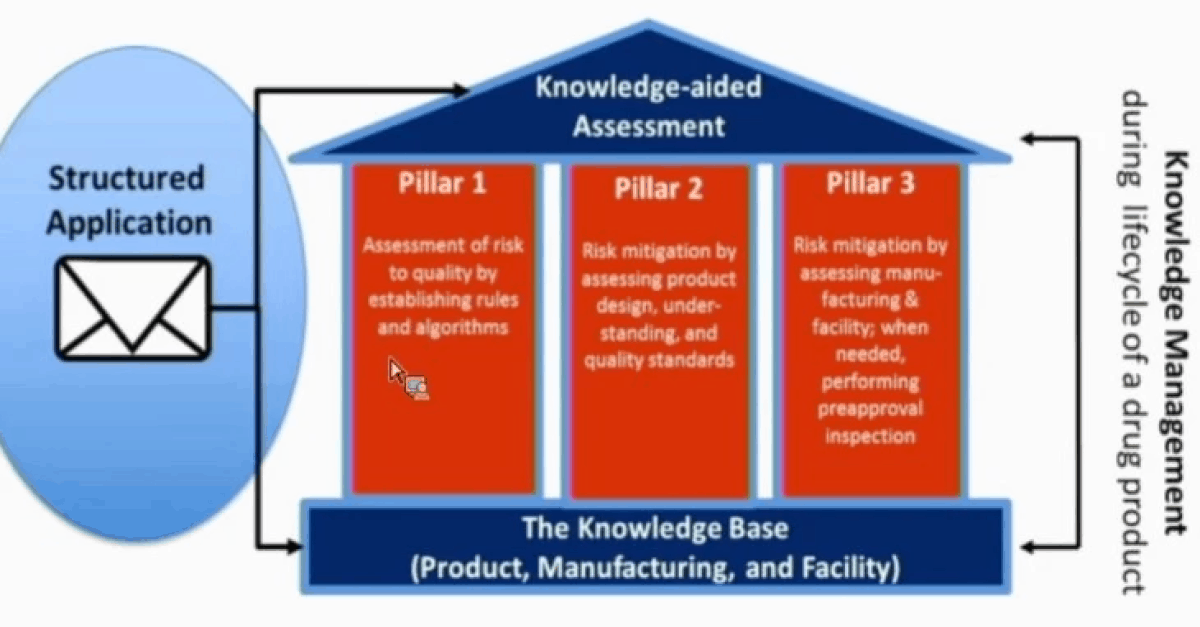
FDA Publishes New Paper on KASA: Why Knowledge Management is Critical to the Drug Industry
Luke Guerrero
May 25, 2019
Last week the FDA published a paper on its pharmaceutical quality assessment system: Knowledge-aided Assessment & Structured Application (KASA) in the International Journal of Pharmaceutics: ...
Read more
CherryCircle Software Named One of Austin’s ‘Most Innovative’ Startups
Luke Guerrero
May 24, 2019
We are proud to announce we’re recognized as one of Austin’s most innovative homegrown companies by the Austin Chamber of Commerce. We are just one ...
Read more
Announcing Our Compliance with 21 CFR Part 11 and ISO 27001
Luke Guerrero
May 2, 2019
Demonstrating compliance with these industry standards and regulations validates that the QbDVision team has implemented comprehensive practices that protect our users, their data, and their ...
Read more
Emma Cartmell Joins the Board of Directors for CherryCircle Software
Luke Guerrero
March 18, 2019
Proven digital health innovator and investor to help advance the Company’s mission to reshape the pharmaceutical manufacturing landscape
Read more
CEO Yash Sabharwal to Speak at Quality-by-Design Symposium in UK
Luke Guerrero
March 7, 2019
We're excited to announce our CEO and co-founder, Yash Sabharwal, Ph.D., will be speaking at the QbD Symposium at De Montfort University in Leicester, England ...
Read more
See CherryCircle Software at ISPE Quality-by-Design Symposium
Luke Guerrero
March 3, 2019
As a Gold Sponsor we are proud to support this academic program as it seeks to advance the application of quality-by-design principles to improve process ...
Read more
CherryCircle Software Raises $2.3M to Advance QbDVision™ for Pharmaceutical Industry
Luke Guerrero
January 29, 2019
The round included participation from ATX Seed Ventures, PLH Business Ventures, Hudson Park Capital, and several industry veterans.
Read more
CherryCircle Software Releases QbDVision for Pharmaceutical Process Development and Manufacturing
Luke Guerrero
September 19, 2018
We're excited to to introduce the new Americana release of our QbDVision process and knowledge management software. Learn more about the platform.
Read more
Harness your expertise
See how QbDVision can help you leverage your team’s know-how to accelerate drug development.