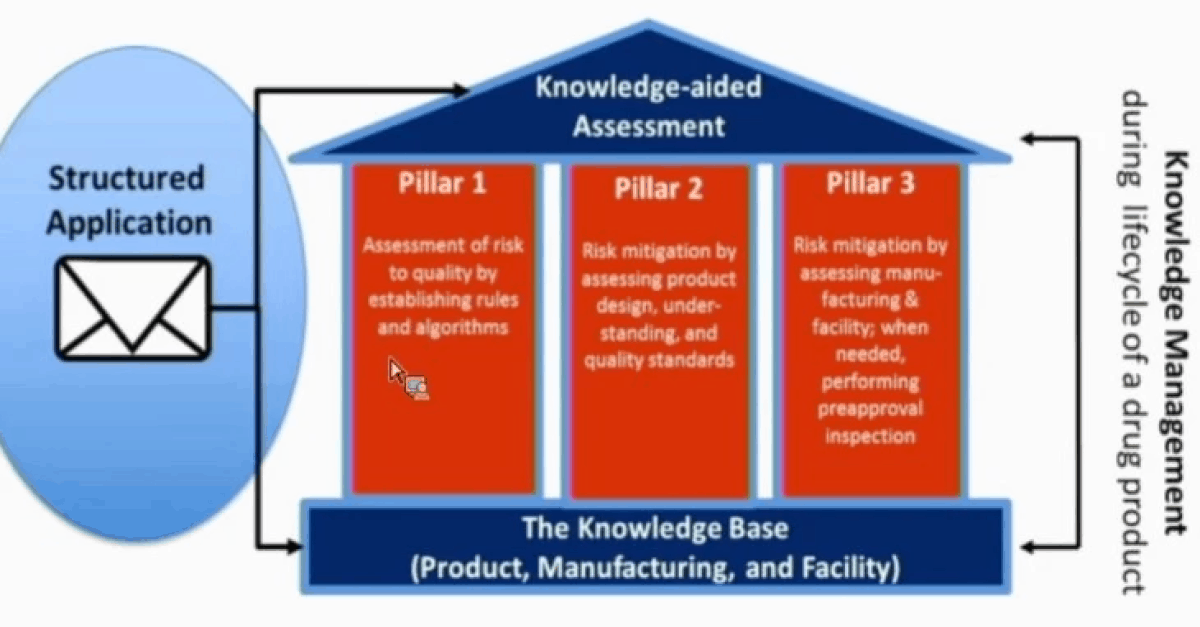
Last week the FDA published a paper on its pharmaceutical quality assessment system: Knowledge-aided Assessment & Structured Application (KASA) in the International Journal of Pharmaceutics: X. With our focus on QbDVision, we see KASA as a significant advancement by the FDA and believe its goals and structure reveal a model which can be replicated for overcoming current industry challenges with quality and cost.
Background on KASA
Last year Dr. Janet Woodcock, Director, Center for Drug Evaluation and Research (CDER) first shared that the FDA was prototyping KASA at the annual meeting of the US Association for Accessible Medicines in Orlando, Florida. In June of 2018, KASA was formally announced by former FDA Commissioner Scott Gottlieb as a program to “modernize generic drug review from a text-based to a data-based assessment”. The program requested $37.6 million and 5 FTEs for a “one-time platform investment” that would “result in more consistent drug product evaluations and seamless knowledge management across generic and brand-name drugs”. Both of these announcements were significant and drew media attention, in part due to the Agency publicly acknowledging its challenges with submissions.
In September of 2018, we were encouraged by the FDA’s discussion at the Pharmaceutical Science and Clinical Pharmacology (PSCP) Advisory Committee, where the committee unanimously agreed (10-0) upon the need for KASA. Just one day before the meeting, we launched QbDVision to the market after extensive development. As you can imagine, we were thrilled to learn how closely their thinking aligned with ours.
We suspect things are accelerating internally following last week’s publication and a recent announcement that the agenda for the upcoming 4th FDA/PQRI Conference on Advancing Product Quality will now include a Special Plenary Session to discuss KASA. We’ll be at the meeting in person and are excited to hear an update.
Why is KASA important?
KASA highlights one of QbDVision’s key observations on the pharma industry: we’ve outlived the age of the document. Here are the FDA’s words directly.
“The lengthy unstructured text narrative with dispersed information and the lack of efficient knowledge management make it difficult for the FDA to compare relative quality and relative risk across drug products and facilities. This makes it difficult to capture the ‘state of quality’ for a product at any given time. This becomes especially evident when assessing residual risks with post-marketing quality changes during the drug product lifecycle. These challenges may lead to late interventions to prevent or address drug shortages or quality failures of marketed drugs. To meet the above challenges, the FDA is developing the KASA system to modernize the quality assessment of drug applications to include structured information. This will promote consistency and enable a much-needed knowledge management tool that will improve efficiency and the overall quality assessment process.”
The agency further highlights three key challenges with today’s Quality-by-Documentation approach:
- “When a quality assessor picks up a regulatory application, it is not possible to easily locate historical data about similar products, processes, or the facilities.”
- “Risk assessment and control can be subjective, leading to inconsistency in regulatory actions and outliers in risk when compared to similar products.”
- “The assessment is a freestyle text narrative that often includes exhaustive documentation of information already provided in the application, and the assessments rely heavily on the knowledge and expertise of the assessor.”
Said simply: if we want to improve the quality of new drugs and speed to market, we must adopt a knowledge-based approach.
And, despite best efforts, today’s approaches are anything but that. Organizations that go out of their way to be thorough in their testing and approach ultimately lose connectivity and traceability to their information, especially when it ends up in a free-form document. On the other side of the equation: the FDA sifts through endless pages of information when reviewing documents in order to understand the drug application.
In today’s time-strapped world, it’s the document that’s holding us back.
What’s new with KASA?
KASA is a new paradigm for performing quality assessments of applications. It’s a move toward a structured assessment rather than a text document. It allows for knowledge management throughout the product lifecycle AND higher efficiency processing by the FDA.
Conceptually, the system’s three core pillars remain unchanged. We performed a structural analysis of the content between the publication and the PSCP September document and found only minor changes following two themes:
- More formal linkages and references to past publications, for example, linking to Q8(2) Pharmaceutical Development for Quality-by-Design.
- More positive or moderated language. For example, calling for “structured assessment that minimizes text-based narrative” as opposed to one that “radically eliminates text-based narratives”.
Based on the information made available to date, it appears that KASA will be a tool used by FDA regulators, not the industry itself. The industry will most likely be impacted by KASA through new FDA submission protocols and data formats.
Overall, the paper pushes KASA along the open publication route that other FDA standards have undergone and serves as an important milestone.
Predictions about KASA
While we have no inside information as to how KASA will progress, as industry observers and builders of the first commercial knowledge management tool for pharma, we offer four predictions to help further shape the conversation.
- Generics as a starting point. The past two administrations have pushed the FDA to promote generic drug entry as a way to foster competition and lower drug prices. And, as noted above, KASA funding comes from an initiative tied to promoting generics. Therefore, we expect the first pathways for using KASA will be with generic applications, but further predict that will quickly expand. The definition and scope of KASA so far appear to be intentionally broader from the onset.
- QbD at the core. At the heart of any knowledge management system are its organizing principles of information. For KASA, we suspect that to be the Quality-by-Design framework adopted by the FDA. KASA publications so far are strongly-linked to patient-centered approaches that are inherent to the framework. QbD seems like a perfect fit for the job.
- Data integration will be tricky. Disparate commercial systems, to which many are homegrown, and vast data organization principles out in the wild will make getting data integration right tricky. We’ve seen this in practice today across the industry – data is all over the place. And, given KASA’s rule and algorithmic based assessments, this part will be all the more important to get right. The publication directly addresses this, referencing calls for unification in Pharmaceutical Quality/Chemistry Manufacturing and Control (PQ/CMC) data and terminologies and eCTD. We’ve previously shared that we feel eCTD has outlived its usefulness, and recent delays add insult to injury. We hope that the industry will join forces here to help drive standardization and normalization around common data.
- Internationalization. In September of 2018, the PSCP Advisory Committee recommended engaging the international community on KASA, similar to other frameworks (e.g., QbD). Publishing a paper is the first step in that process. We know the topic is being discussed internationally. For example, this week our CEO, Yash Sabharwal, is in the UK speaking on the topic of structured knowledge management at De Montfort’s Quality by Design Symposium.
What happens next?
We hope to hear a lot more about KASA this year. We’ll be active participants at the 4th FDA/PQRI Conference on Advancing Product Quality next month, where the KASA initiative will be outlined by an expert FDA panel moderated by Dr. Lawrence Yu, Deputy Director of CDER’s Office of Pharmaceutical Quality, along with distinguished presenters from the FDA including Dr. Susan Rosencrance.
Explore what knowledge management can do for you
QbDVision is the first cloud-based, Structured Knowledge Management Solution (SKMS) for the Pharmaceutical industry. Unlike KASA, it’s built for the industry (by industry insiders). QbDVision is available today for your exploration. Sign-up for our Sandbox environment and explore example case studies, or try your own project. Contact us directly or use the feedback button and tell us what you think.