In an age of digital transformation, the life sciences still struggle with an age-old challenge.
For businesses capable of scientific marvels, pharma and biotech companies have a bewilderingly hard time moving information and processes from one place to another.
Tech transfers – an inevitable and recurring step in every drug development pathway – continue to bedevil the industry, bloat timelines, strain budgets, and delay the launch of promising new therapies. Today it takes 24-30 months on average to transition a product and its processes from one site to another, and personnel costs alone have ballooned to $38-389MM per transfer.
So how has this essential step been left behind by all the innovation that has come to the industry’s discovery, clinical development, and commercial functions?
There are many theories on why tech transfers are particularly resistant to transformation. But as a major QbDVision customer realized, the real answer starts with a question: How can you efficiently transfer knowledge and information when you don’t know where it is, who has it, or what format it’s going to be in?
Solving the tech transfer conundrum
Slow, costly, error-prone tech transfers are often one of the biggest issues drug developers are facing when they come looking for a solution like QbDVision. By the time this customer onboarded our technology, they were already struggling with some of the most common causes of that challenge.
One of the most prominent producers of generic medications, our customer operates multiple manufacturing facilities across the globe and has an established presence in over 20 markets worldwide. But while they had big plans to extend their growth, they had been hampered by several key problems that were all too familiar to our team:
- Unstructured data: Lack of consistent organization and taxonomies made it difficult to leverage their wealth of existing knowledge.
- Paper-based systems: Reliance on hard copies left most of the organization with limited access to even their own projects’ information.
- Limited collaboration: Departmental silos added to the challenge of sharing unstructured information locked in physical formats.
Why were tech transfers so challenging ? The real answer starts with a question: How can you efficiently transfer knowledge and information when you don’t know where it is, who has it, or what format it’s going to be in?
Every one of these limitations was a roadblock in their path to the “right-first-time” development challenges the company wanted to implement. We stepped in to help them remove the cost-driving, time-consuming friction in multiple areas of their business – but especially in their tech transfer processes.
Connecting scattered teams, data & processes with a unified knowledge platform
Data decentralization is an endemic problem in the life sciences, so our customer’s challenges were ones our team had seen many times before. Over the next 18 months, we made it our mission to modernize the structure, exchange, and utilization of data across this busy organization and its workflows.
Over 200 active projects needed to be transferred from a 100% paper-based system to our digital knowledge base – no easy task for us or them. Together, we started the journey with a single pilot project and a few critical goals:
- Craft our customer’s vision for transformation, including clear digitalization objectives.
- Identify key stakeholders in their development processes.
- Assess their current processes and available data to pinpoint gaps in essential information.
- Establish a consistent approach to digitizing their analog workflows.
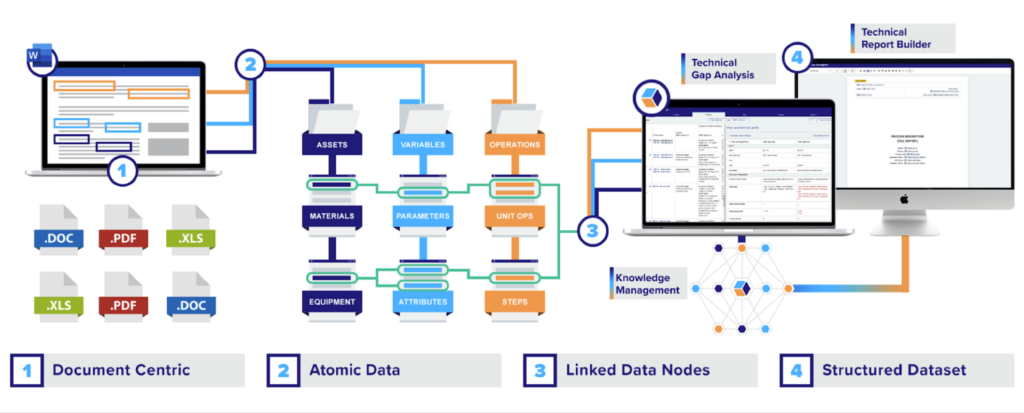
This initial collaboration gave our team the insights we needed to create a systematic plan for centralizing this knowledge in QbDVision’s unified data framework. The first step: deploying a 4-step Digital CMC process that would put them on the path to measurable improvements in their business performance.
Implementing Digital CMC: The power of Atomize > Structure > Connect > Leverage
At the heart of our customer’s struggles was one of the industry’s hallmark challenges: reliance on documents. Their paper-based systems locked vital information in static, isolated files, while indexing these documents – instead of the data in the documents – added friction to every workflow that relied on that information.
To help them break out of that trap, we deployed a road-tested process for transforming CMC operations:
1
2
3
4
Once that process was complete and a unified knowledge base was in place, our customer could immediately see the benefits in multiple key areas of their business:
Accelerated tech transfers
Initially, they struggled with many of the hallmarks of conventional tech transfer processes – including fragmented hand-offs to MS&T and QA, gap analyses hampered by document silos, and tedious risk recalculations at different process scales.
But with their newly centralized knowledge base, they could switch to QbDVision’s Digital Tech Transfer workflow. Our solution enabled them to conduct side-by-side gap analyses, visualize their risks, and streamline knowledge exchange across every site and scale.
The result: Far fewer headaches and months less work.
Supercharged report generation
Like scientists anywhere, experts at this organization weren’t thrilled to be spending the bulk of their time digging raw data out of spreadsheets and manually entering it into sprawling documents. It took them challenging amounts of time to generate reports like the FMEA.
That changed quickly once we centralized our customer’s knowledge and data in QbDVision and fully connected each datapoint to relevant attributes, parameters, and components. In QbDVision’s 21 CFR 11 compliant infrastructure, their scientists could generate reports like the FMEA – fully approved and signed-off – in a matter of hours. Not weeks.
Now that they’ve shrunk their reporting timelines by up to 95%, we don’t see manually wrangling data in their future any time soon.
Visualized risk assessment
With their data consolidated, structured, and contextualized in QbDVision, our customer could visually map potential impact chains throughout their development strategies. This unique feature significantly enhanced their risk assessment and management practices by making it easier to spot the correlation of input and output variables, as well as the impact of changes, variances, and nonconformities.
QbDVision has allowed us to create a standardized framework for development, from R&D to Manufacturing, based on the holistic principles of Quality by Design, FAIR Data, and the ICH Guidelines. Enabling myself and my colleagues in R&D, MS&T, QA, and Project Management to collaborate together within a unified knowledge platform.
Lead Process Engineer
Streamlined submissions
In assessing the data management practices within this organization, we quickly found that they had fallen into an all-too-common bad habit: tracking the same data points in multiple different documents in numerous different locations. As a result, supposedly simple updates to unit operations, process parameters, and quality attributes frequently meant tracking down 5-10 different documents across the organization.
Not surprisingly, this repetitive, time-consuming process often led to data integrity issues and operational inefficiencies, especially when information was captured freestyle in narrative-based documents. They were badly in need of a centralized source of truth where data could be stored, indexed, and exchanged with anyone who needed it – which is exactly what QbDVision provided.
With Doc Builder, templated submissions like the eCTD Module could be easily and directly generated from their centralized, structured knowledge base. Their CMC teams could fly through the pharmaceutical development component of the eCTD*, as well as other key inputs relating to Finished Product Specifications, Control of Critical Steps and Intermediates, Manufacturing Equipment, and the Manufacturing Process.
*Section P.2 of the eCTD Module 3: Outlines the underlying knowledge that establishes the developed formulation and chosen dosage form that are suitable for their intended use.
SPOTLIGHT
QbDVision's Doc Builder
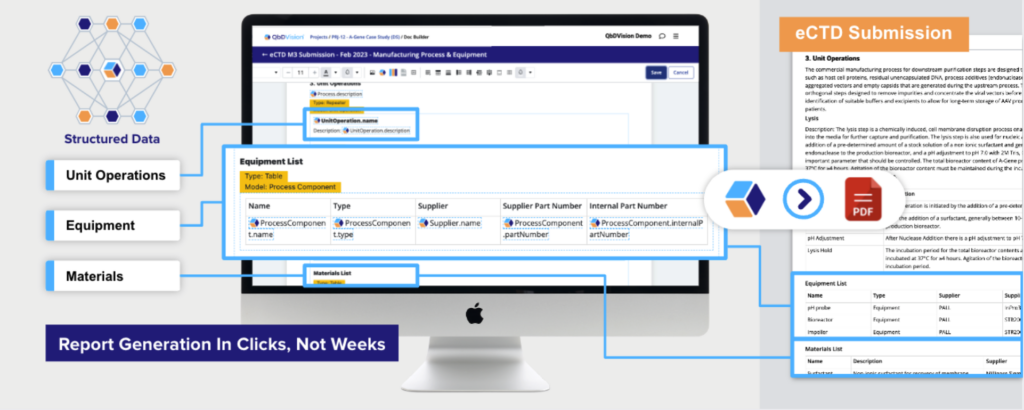
Create, maintain, review – all in one place
One of users’ favorite QbDVision features, this powerful tool harnesses the power of your data to dynamically create reports in a fully compliant, validated environment.
- Automate generation of templated reports like FMEA and eCTD Module 3
- Customize outputs to your organization’s style and taxonomies
- Eliminate tedious data consolidation and entry steps
What everyone wants to see: Tangible, measurable business outcomes
For almost 2 years now, QbDVision has been critical to the broader digital transformation strategy – and has delivered some impressive results.
↓95% reporting time
QbDVision’s automated reporting capabilities eliminated duplicate work on FMEA and other development reports. One key reporting task was reduced from 3 weeks to just 6 hours.
Simplified scale-up, process optimization & post-approval change management
Instead of updating several documents in various locations, their CMC teams can eliminate repetitive work and reduce cycle times by updating centralized data for on-market products.
Data-driven audits streamlined by a single source of truth
Auditors can simply log in to QbDVision to get a fully contextualized understanding of both product and process requirements.
3X faster tech transfers
The Digital Tech Transfer workflow accelerated handoffs from R&D to manufacturing, while integrated Transfer Risk eliminated time-consuming risk recalculations at different scales.
Risk management & assessment enhanced by risk-based traceability
Accelerated development of eCTD Module 3 submission
A centralized, validated knowledge hub reinforces data integrity and keeps knowledge from being lost in freestyle, narrative-based documents.
↓95% reporting time
QbDVision’s automated reporting capabilities eliminated duplicate work on FMEA and other development reports. One key reporting task was reduced from 3 weeks to just 6 hours.
3X faster tech transfers
The Digital Tech Transfer workflow accelerated handoffs from R&D to manufacturing, while integrated Transfer Risk eliminated time-consuming risk recalculations at different scales.
Simplified scale-up, process optimization & post-approval change management
Instead of updating several documents in various locations, their CMC teams can eliminate repetitive work and reduce cycle times by updating centralized data for on-market products.
Data-driven audits streamlined by a single source of truth
Auditors can simply log in to QbDVision to get a fully contextualized understanding of both product and process requirements.
Risk management & assessment enhanced by risk-based traceability
Accelerated development of eCTD Module 3 submission
A centralized, validated knowledge hub reinforces data integrity and keeps knowledge from being lost in freestyle, narrative-based documents.
↓95% reporting time
QbDVision’s automated reporting capabilities eliminated duplicate work on FMEA and other development reports. One key reporting task was reduced from 3 weeks to just 6 hours.
3X faster tech transfers
The Digital Tech Transfer workflow accelerated handoffs from R&D to manufacturing, while integrated Transfer Risk eliminated time-consuming risk recalculations at different scales.
Simplified scale-up, process optimization & post-approval change management
Instead of updating several documents in various locations, their CMC teams can eliminate repetitive work and reduce cycle times by updating centralized data for on-market products.
Data-driven audits streamlined by a single source of truth
Auditors can simply log in to QbDVision to get a fully contextualized understanding of both product and process requirements.
Risk management & assessment enhanced by risk-based traceability
Accelerated development of eCTD Module 3 submission
A centralized, validated knowledge hub reinforces data integrity and keeps knowledge from being lost in freestyle, narrative-based documents.
Looking ahead, this customer was planning to extend their paper purge by implementing an eQMS – a complementary system that can fully eliminate their paper-based document management, training management, and supplier management workflows.
Today, as their digitalization gathers momentum, they’re continuing to migrate their remaining projects to QbDVision. We’re excited and proud to see their ongoing commitment to our platform, Digital CMC, and ultimately, to accelerating the delivery of high-quality, life-changing therapies to patients.
GET IN TOUCH
Want to see results like these on your CMC projects?
Reach out to our team of experts to learn more about what made this collaboration a success – and how we can help put your drug development team on the path to Digital CMC.