DIGITAL CMC SOLUTIONS
Streamline your tech transfers
Discover how QbDVision turns one of your biggest operational bottlenecks into an efficient, modern digital workflow.
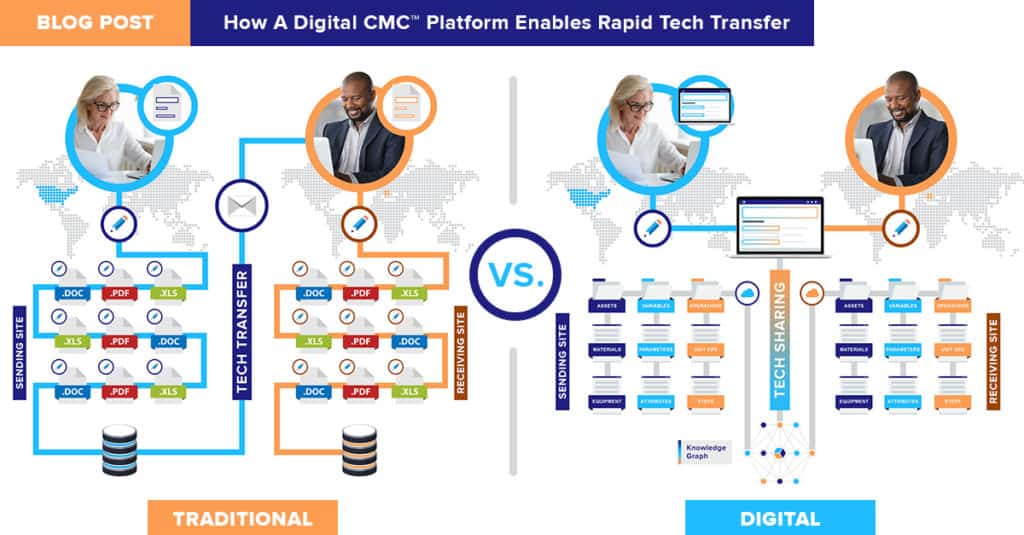
Today’s tech transfers are slow, expensive, and document-dependent
Exchanging knowledge and data is the crux of this critical process. But all too often, those vital resources are locked in PDFs, buried in inboxes, or stacked in folders and SharePoints.
Manually gathering, sifting, and sharing documents is a time-consuming, costly process that bottlenecks development – and not just once, but many times in your product’s life cycle. It’s time for a better way to transfer.
24-30 Months
on average to complete a tech transfer
$38-389MM
in personnel costs alone for a typical 28-month tech transfer
80%
of scientists’ time is spent sourcing and consolidating information
We turn documents into usable, sharable, real-time process data
Never chase another file or get lost in another SharePoint. QbDVision is the source for all your CMC resources – QTPPs, CQAs, CPPs, analytical methods, change control logs, and more – your organization’s data is unlocked, connected, and accessible to your entire transfer team.
It’s all there for everyone, in an insight-rich process hub that makes tech transfers faster and more efficient than ever.

With fast, efficient data exchange, everybody wins
Make sharing knowledge as simple as logging in
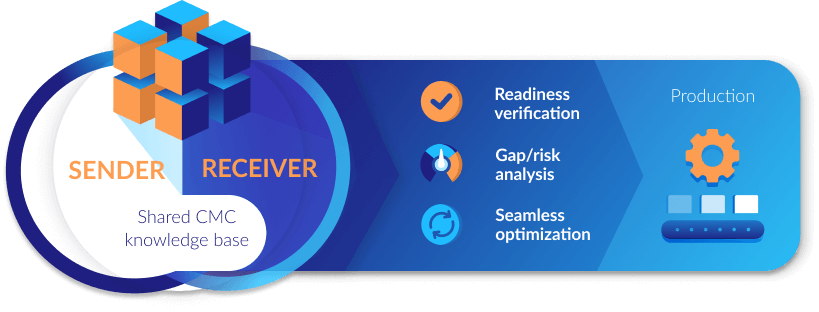
Verify transfer readiness
Instantly analyze gaps & risks
Optimize seamlessly
Simplify handoffs with structured deliverables
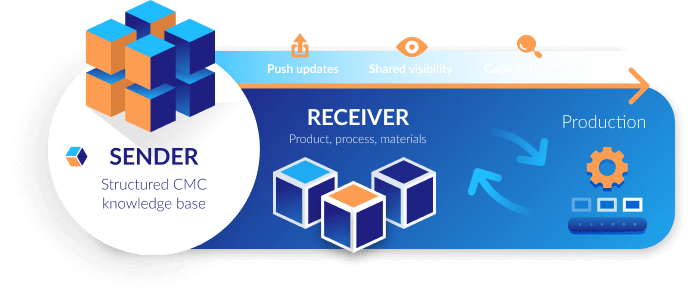
Lower operational overhead
Trace every development step
Provide real-time visibility
Create a master structure for every transfer

Protect 21 CFR compliance
Master change management
Simplify scale-outs
Make sharing knowledge as simple as logging in

Verify transfer readiness
Instantly analyze gaps & risks
Optimize seamlessly
Create a master structure for every transfer

Protect 21 CFR compliance
Master change management
Simplify scale-outs
Create a master structure for every transfer

Protect 21 CFR compliance
Master change management
Simplify scale-outs
All your teams, data, and workflows. All in one dynamic workspace
Sending or receiving, successful tech transfers demand cross-functional collaboration. QbDVision makes that teamwork easier than ever.
Our platform gives your whole team a secure, centralized source of truth for all their critical process, product, materials, and risk information. Sharing data update, analyzing risks, contextualizing changes – in QbDVision, it’s easier than ever. For everyone.

Tech transfers, meet state-of-the-art transfer technology

End-to-end security
Keep your IP safe and your data compliant with best-in-class cybersecurity and access management protocols.
Modern data management
Turn your documents, folders, and SharePoints into productivity- and value-enhancing business resources.

Enhanced productivity
Free your team from from documents and get your scientists, engineers, and process experts back to what they do best.

After completing numerous tech transfers with QbDVision, I can’t imagine initiating one without it. It saves us time, money, and headaches at every step, even with complex biologic products.
OUR LATEST
Dive deeper with our CMC thought leaders
MORE INSIGHTS
Take a deep-dive with QbDVision Co-Founder & CEO, Yash Sabharwal.

MORE INSIGHTS
Explore new best practices and technology solutions with our CEO Yash Sabharwal and COO Luke Guerrero.
See the transformation for yourself
Schedule a consultation with our tech transfer experts today.






















































