OUR PLATFORM
Discover all your knowledge can do
Start with the software that brings digital chemistry, manufacturing, and controls (CMC) to pharma and biotech for the first time.
Digital CMC for drug products
In an industry full of retrofit software solutions, QbDVision is a purebred platform for pharma and biotech – – purpose-built to deliver the solutions that matter most for teams like yours.
your unstructured knowledge and data
repetitive tasks and complex workflows
across teams and functions
exactly how your projects and risks are evolving
your data and ensure it’s regulator-ready
development of new breakthrough therapies
OUR FOCUS
Solving a legacy challenge
Today, lack of structure slows development
Our industry still leaves its knowledge and data scattered across inboxes, folders, spreadsheets, PDFs, and legacy QMS. The result: inefficient processes, poor process understanding, product quality risks, and product life cycles delayed by information barriers.
Our structured platform brings it all together
QbDVision provides a unified framework that connects it all — your information, your processes, and your people — in ways that help teams turn science into products with unprecedented efficiency, coordination, and agility.
WHAT YOU GET
One software with all the tools you need
With QbDVision, they can manage everything from a single login — process evolution, risk evaluation, and data analytics — without the inefficiency and data integrity risks of disconnected, legacy software solutions that create barriers to shared knowledge.
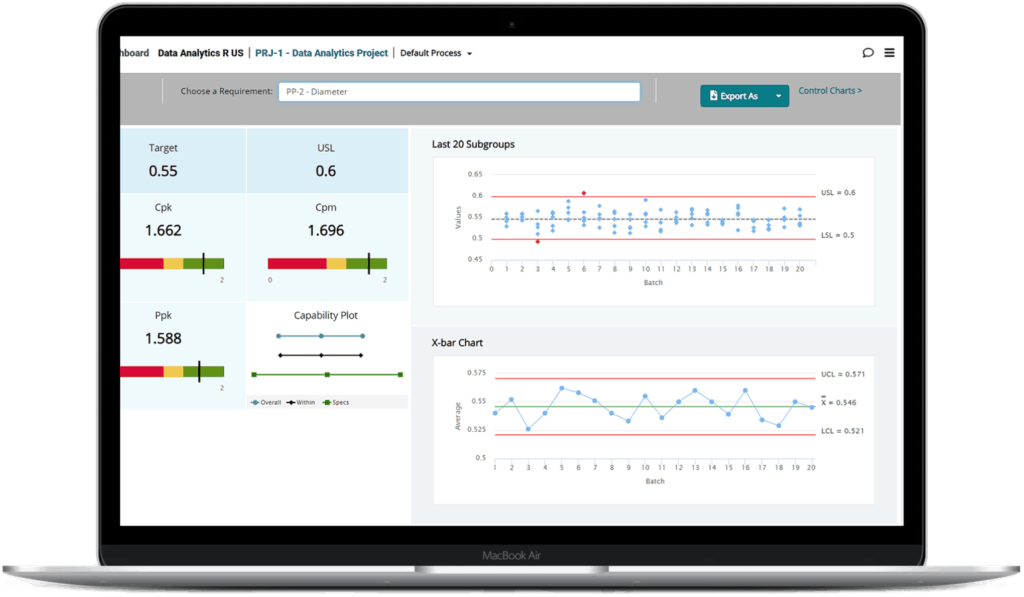
WHAT YOU GET
One software with all the tools you need
With QbDVision, they can manage everything from a single login — process evolution, risk evaluation, and data analytics — without the inefficiency and data integrity risks of disconnected, legacy software solutions that create barriers to shared knowledge.

WHAT QbDVISION CREATES
A foundation for simpler workflows and smarter decisions
With all your knowledge, data, and systems in one place, you can finally sync up all the workflows that depend on those resources. And with all those activities in sync, it’s easy to see how you can optimize every one of them.

Solve fragmentation
Unite siloed workflows, reveal implicit knowledge, and collect scattered data.

Consolidate information
Create a shared, centralized knowledge base with a structured framework.
Enhance coordination
Make it easy for your teams to work together, align approaches, and transfer knowledge.

Create intelligence
Make every decision based on contextual guidance and visualized insights.
WHAT IT DELIVERS
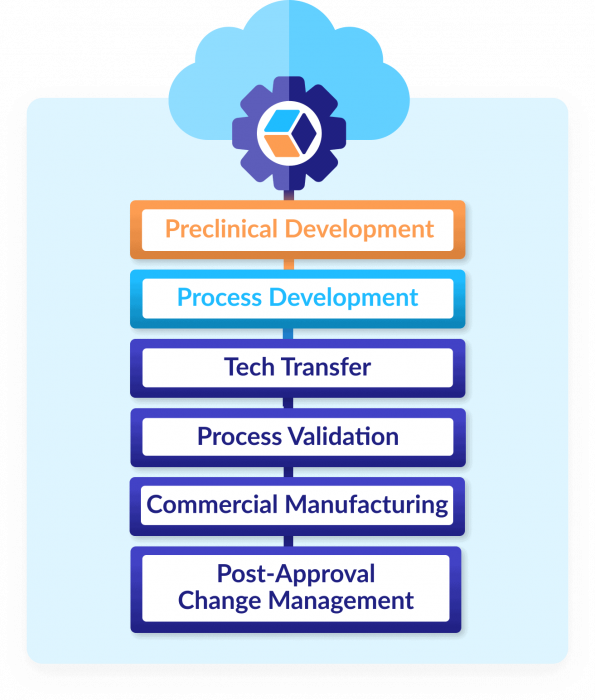
A modern digital path to efficient, agile CMC
As your product and processes evolve in QbDVision, so do your understanding and control. Using our platform ensures you always have a holistic, historical view of the entire life cycle — so you can always make the best decision at exactly the right time.
KEY FEATURES
Benefits of a structured platform
Fast deployment
Your teams can be up and running on our cloud-based software in minutes. With our intuitive user interfaces, inline documentation, and world-class customer support, your teams can bring down data silos and start building a shared knowledge base in a few clicks.
Built-in compliance
Every click in QbDVision facilitates compliance with ICH and ISO quality guidelines, 21 CFR 11, and EU Annex 11 data integrity requirements. Users and regulators can easily access the complete, accurate, and fully contextualized information they need at every step of the product life cycle.
Robust security
GO DEEPER
See everything our platform makes possible
KNOW WHERE
Centralized knowledge management
Collect all your teams’ data and knowledge in one searchable, cloud-based hub.
KNOW HOW
Streamlined process development
Create robust, agile processes that your teams can control as one.
KNOW WHY
Powerful process intelligence
See what every action means for the whole product life cycle.






















































