QbDVision plans
See what’s included in our Essentials and Professional plans, and find out which one’s right for your drug development teams.
Essentials
Early-Stage/Preclinical
Start your early-stage venture on the right foot with the basics of electronic quality and product lifecycle management.
Starting at
Professional
Process Development
A comprehensive software solution to manage all of your process development activities before starting GMP manufacturing.
Starting at
$229/user/month
Professional-XR
Clinical Manufacturing & Process Validation
A validated version of the Standard offering to manage GMP-level process development and validation activities.
Starting at
$259/user/month
| Features | Essential | Professional | Professional-XR |
|---|---|---|---|
| Document Management |
|
|
|
| Training Management |
|
|
|
| Supplier Management and Risk |
|
|
|
| Target Product Profile |
|
|
|
| Quality Target Product Profile |
|
|
|
| Process Development |
|
|
|
| Process Risk Assessment |
|
|
|
| Raw Material Management |
|
|
|
| Qualification & Testing |
|
|
|
| Process Data & Analytics |
|
|
|
| Control Strategy |
|
|
|
| Data Visualization Tools |
|
|
|
| Automated Reports |
|
|
|
| Validation Package (Add-on) |
|
|
QbDVision Essentials
Start simplifying quality management
Stuck with an inefficient paper-based QMS or expensive, complex eQMS? Start managing quality and compliance more cost-effectively than ever with QbDVision Essentials—at a fraction of the cost of an eQMS.
Who it's for
Lean teams ready for a streamlined eQMS solution
What's included
Core eQMS functionality that your team can start using in minutes
What it does
Digitally integrate quality and product life cycle management workflows
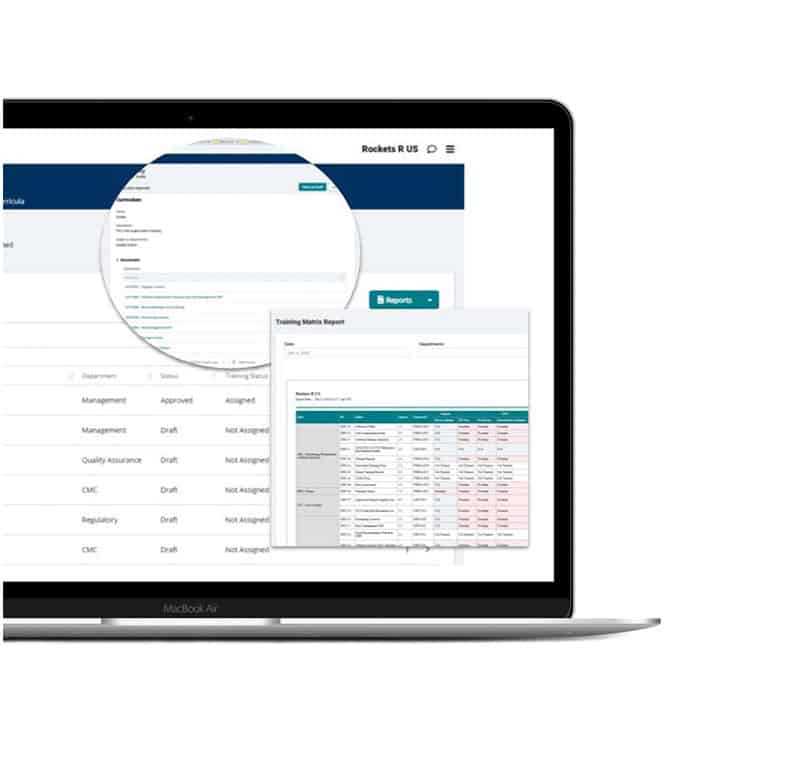
Training integration
Stop managing training from your inbox. Integrate all your user profiles, training plans, curricula, and assignments into one pre-validated solution that instantly brings your program into cGMP compliance.
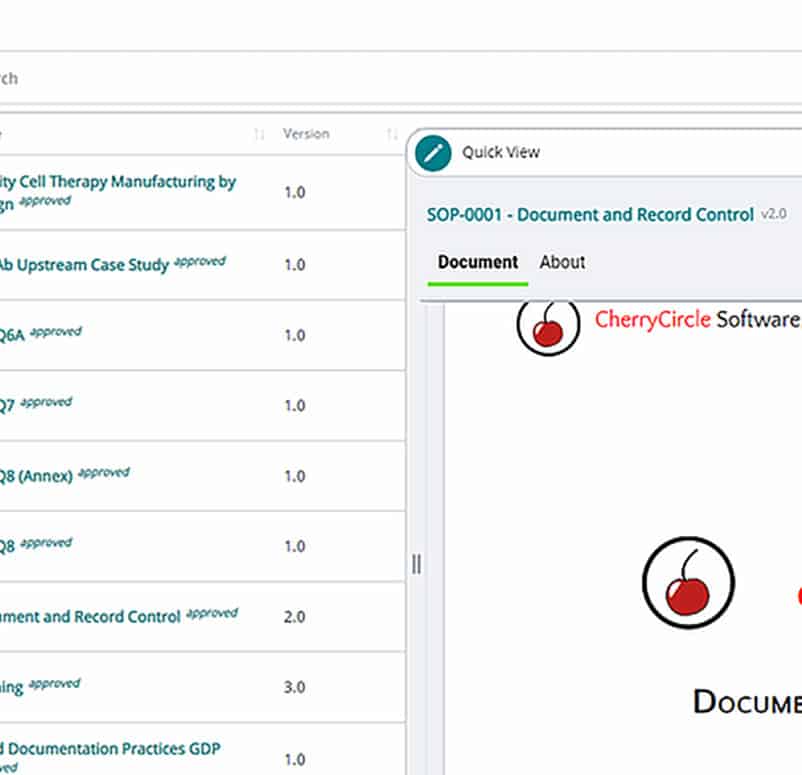
Electronic document management (EDM)
Manage your users, training programs, and documents with a single streamlined workflow. Digitally route, review, and sign documents in compliance with 21 CFR 11, all from one searchable platform.
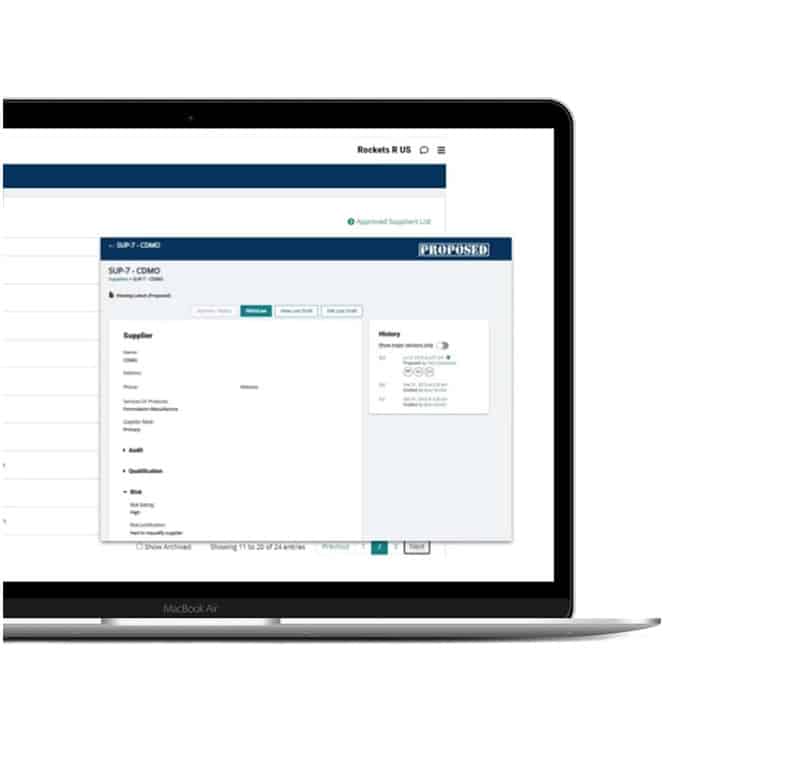
Supplier management
Build a digital GMP supplier database with integrated audit tracking, qualification management, and risk assessment tools. Pull up quality agreements, approved vendor lists, and more in just a few clicks.
QbDVision Professional
Accelerate your product development cycle
Our Professional plan provides full access to QbDVision. Give your team the power to connect their fragmented data, unite their scattered knowledge, and transform it into process intelligence that streamlines drug development.

Who it's for
Growing teams that need a unified framework for their collaboration
What it does
Transforming dispersed data and knowledge into smart, holistic guidance
What's included
Full QbDVision platform and all functionality (non- or fully validated)
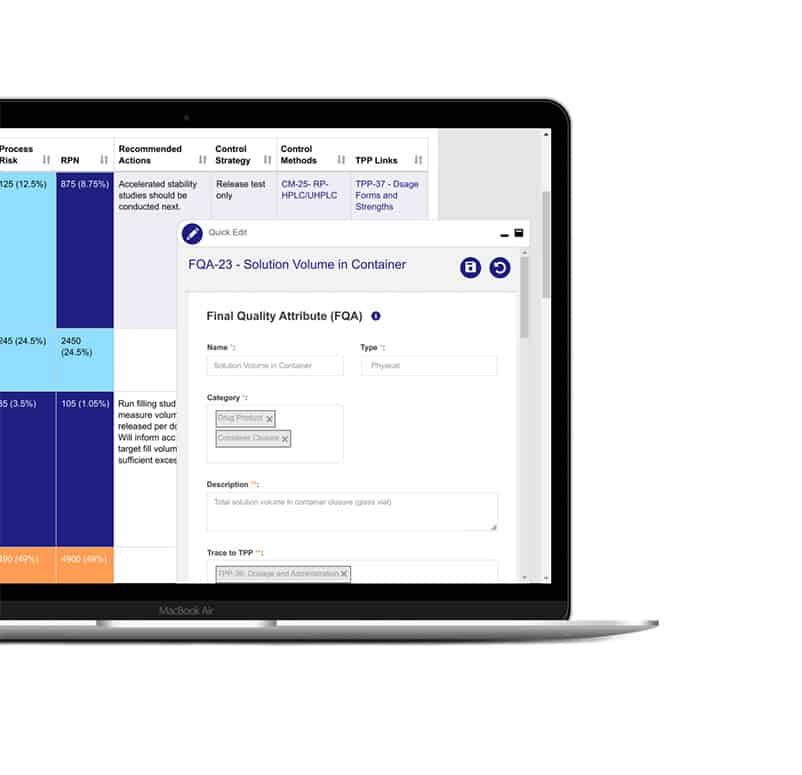
One centralized source of truth
Consolidate all your teams’ data and knowledge in one structured, searchable hub where they manage their product’s life cycle with the power and efficiency of a modern software tool.
Agile, efficient development processes
Sync, standardize, and monitor critical workflows with a single platform—one that makes it easy to transfer everything you’ve learned to the next development team and their project.
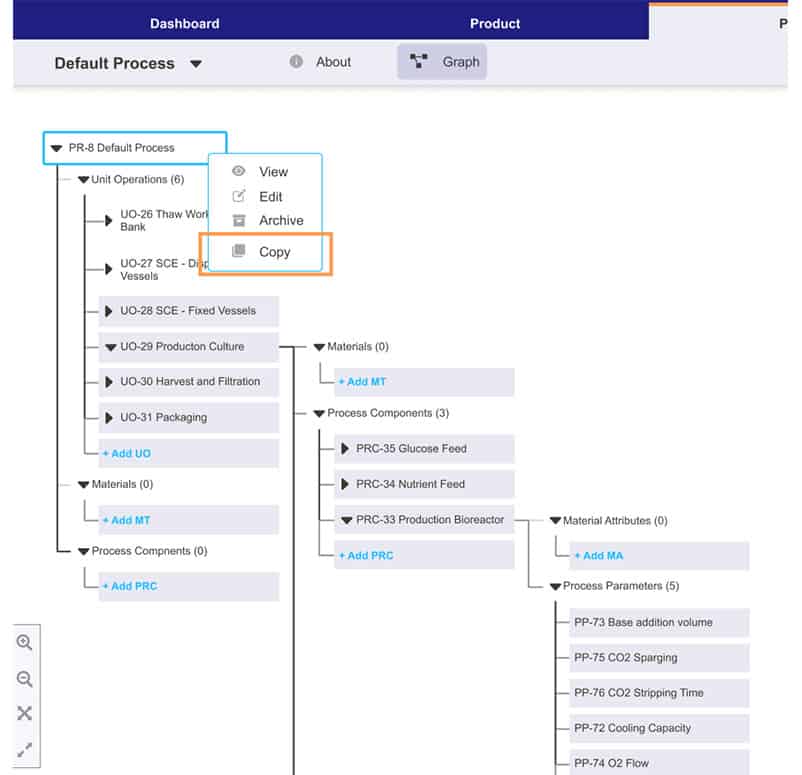
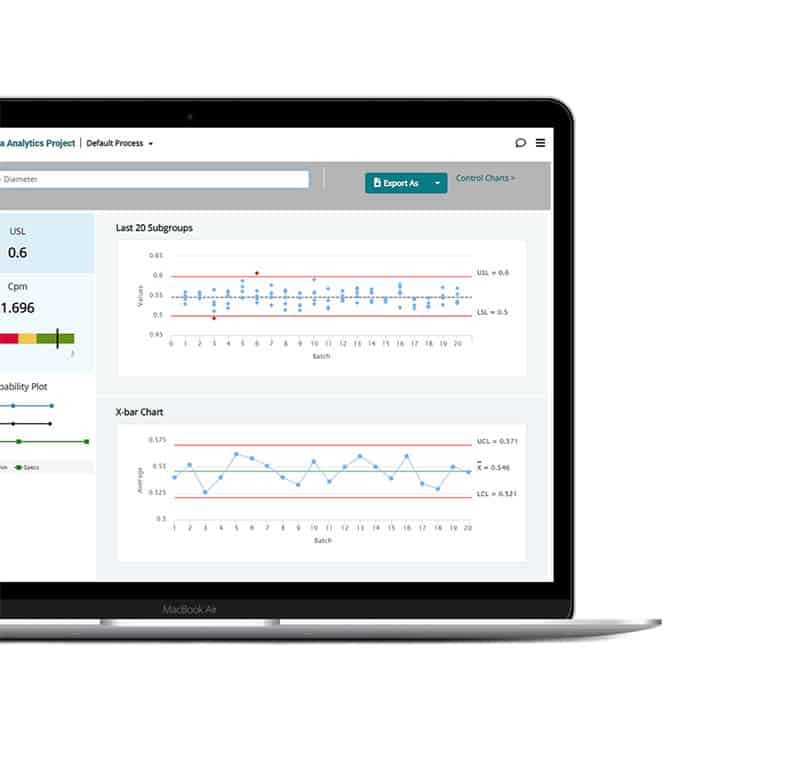
Process intelligence at every step
Turn your teams’ knowledge and date into smart, holistic guidance, timely decision support tools, and powerful visual insights—everything they need to identify the right decision every time.
QbDVision Professional-XR
Our fully validated solution
Want to use your PLM platform as your system of record for managing ICH- and GMP-compliant activities? Our Professional plan is also available as QbDVision Professional-XR, a 21 CFR 11 compliant version of our platform that can have you up and running in a fully validated environment in minutes.
- GAMP3 solution that requires no client-side PQ
- Configuration & Deployment Qualification (CDQ) for cloud-based solution
- Fully-automated operational qualification with full document set, including protocols and results
- Performance qualification for both automated and manual tests, with full protocols and results
See all our plans in action.
Try QbDVision Essentials today, or book a one-on-one demo of QbDVision Professional or Professional-XR.






















































