KNOW WHERE
Centralize your data and knowledge
Consolidate and integrate all your teams’ development resources on a single, searchable platform.
Everything your teams know, all in one place
Drug development processes create astounding amounts of information. But insights? All too often, they stay hidden in static documents or siloed knowledge bases.
Now you can finally consolidate your teams’ wealth of dispersed data and knowledge. And discover what it can really be worth.
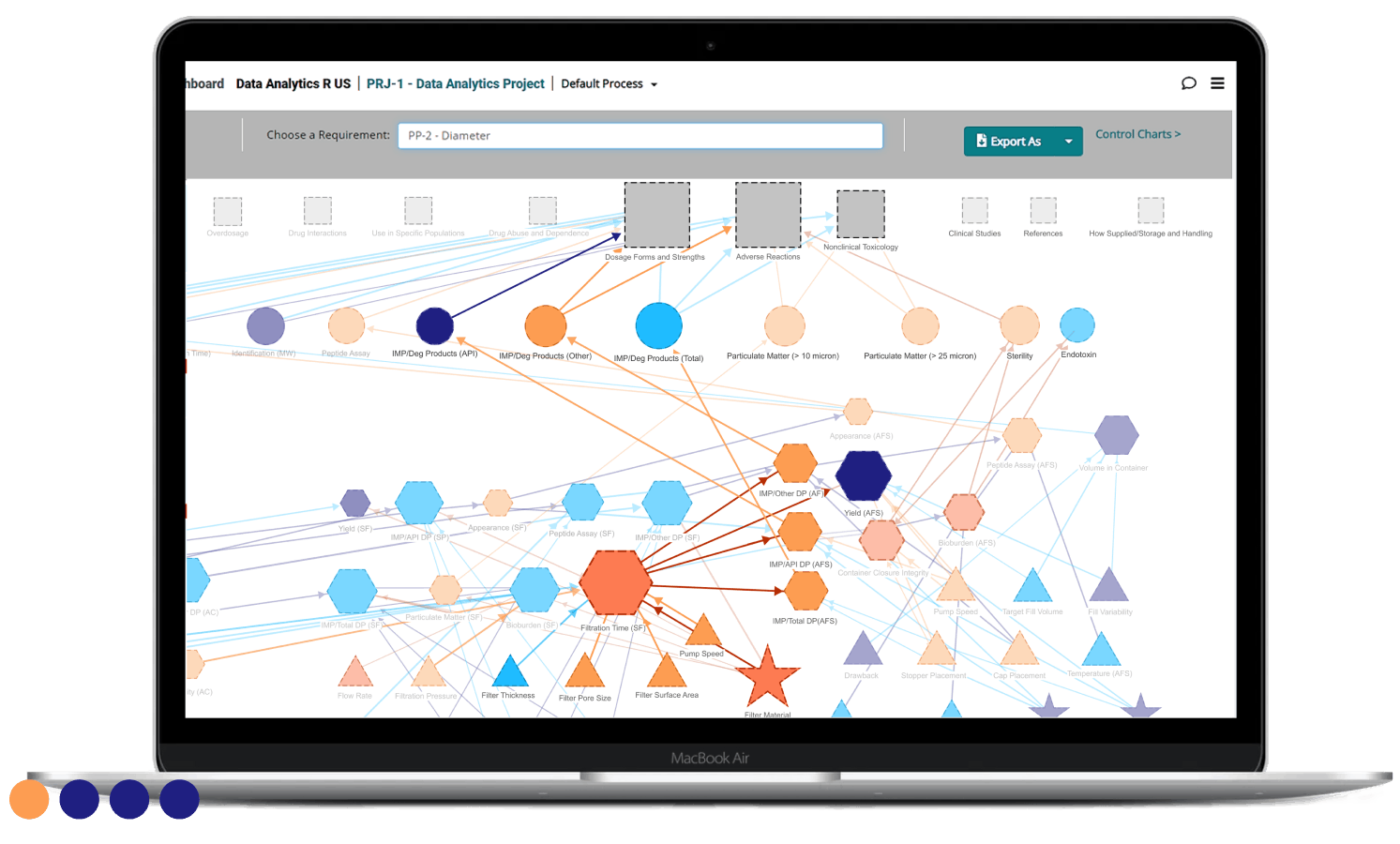
CONSOLIDATE
Create a single, structured source of truth
Give your teams one destination for all the information they need to advance your product’s development, including:
- Patient, product, and process requirements
- Acceptance criteria
- Notes, sketches, and diagrams
- Reports & updates
- Transfer information
CONSOLIDATE
Create a single, structured source of truth
Give your teams one destination for all the information they need to advance your product’s development, including:
- Patient, product, and process requirements
- Acceptance criteria
- Notes, sketches, and diagrams
- Reports & updates
- Transfer information

CONNECT
Manage it all right here
All your teams can finally create, sync, and share their critical project information with one software. QbDVision syncs all their knowledge and data across functions, workflows, and materials, from process, to product, to patient.

UNIFY
Bring everyone together on one platform
Drug development teams are more dispersed than ever. QbDVision gives them a dedicated space to share information, resources, and visibility into each other’s workflows. If it can help them make smarter decisions, avoid redundancies, or better understand their evolving processes, they can find it here.
UNIFY
Bring everyone together on one platform
Drug development teams are more dispersed than ever. QbDVision gives them a dedicated space to share information, resources, and visibility into each other’s workflows. If it can help them make smarter decisions, avoid redundancies, or better understand their evolving processes, they can find it here.

STREAMLINE
Move to an automation-ready framework
Lay the groundwork for automating complex, time-intensive activities. Users can easily create standardized workflows that streamline compliance with industry guidelines and best practices — including analytics, reporting, risk assessments, and more.
FEATURED SOLUTION
Run critical reports with a click
Forget wasting hours writing and updating reports. QbDVision provides the clean, structured data you need to complete this multiweek manual task in minutes.
- Generate and update reports in real time
- Instantly create visualized dashboards
- Create historical lookbacks at any time
GO DEEPER
See everything our platform makes possible
OUR PRODUCT
Modern software for pharma and biotech
See how QbDVision digitizes management of the whole product life cycle.
KNOW HOW
Streamlined process development
Create robust, agile processes that your teams can control as one.
KNOW WHY
Powerful process intelligence
See what every action means for the whole product life cycle.
See it all come together
Find out how easy it is to consolidate all your data and knowledge in one modern digital platform.






















































