Driving Operational Excellence
In this installment of QbDVision’s Knowledge Management Lab, we discuss how developing an internal digital knowledge management capability for your organization can translate into operational excellence based on agile management principles.
We see the objective of achieving organizational agility for CMC, MSAT, and Process Development teams as a critical factor in the future success of the pharma/biotech industry. The path to organizational agility and overall operational excellence starts with knowledge management as part of a broader digitization effort. Empowered teams that are moving quickly and focused on outcomes must share a collective intelligence. Traditional hierarchical approaches to organizational management and information sharing can kill an agile culture. To move fast, teams need a way to collect, enrich, and share information broadly and without delay.
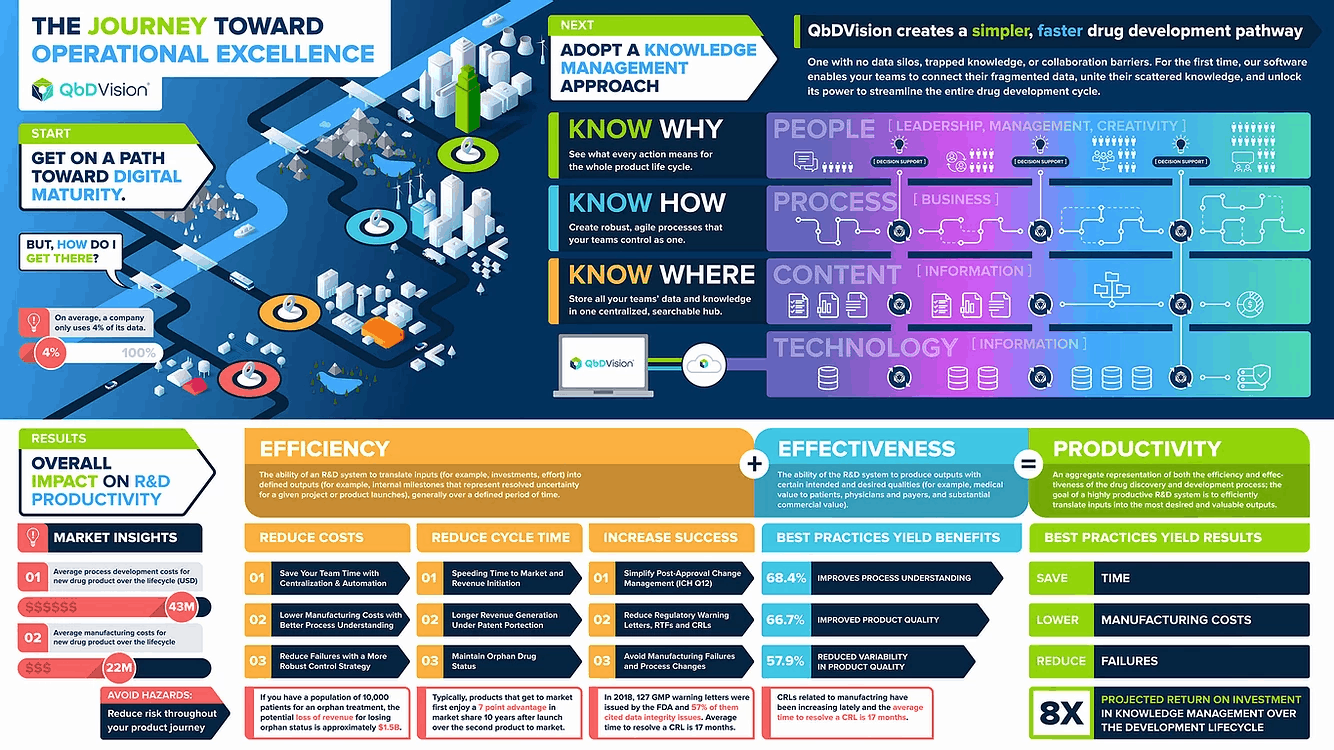
Did you know that pharma/biotech companies use less than 4% of their data? This statistic demonstrates that we have plenty of opportunities for improvement. While knowledge management initiatives can improve data utilization, they have to go beyond simple aggregation of content (“the SharePoint approach”). In this whitepaper, we offer some practical suggestions on how to get started with knowledge management and explain why this approach ultimately improves your process understanding and overall process intelligence.
While there isn’t a one-size-fits-all approach to achieving operational excellence, starting here will provide an actionable path to building organizational excellence and getting higher quality products to patients faster. Something we’re all after.
Start With Knowledge Management
A primary objective of the product development lifecycle is to develop a scalable manufacturing process that is well-controlled and consistent in the level of product quality to ensure patient safety and efficacy. Traditionally, this process has been linear and reactive with a focus primarily on quality compliance. Recent initiatives, such as Right First Time and Pharma 4.0, have focused on re-thinking this development process so that it is proactive, holistic, driven science and data, and defined by digitally-enabled best practices such as Quality-by-Design (QbD). To this end, knowledge management (KM) and its close counterpart, quality risk management (QRM), are seen as key elements of these new initiatives.
This need for systematic knowledge management has gained further traction with the creation of the BioPhorum Knowledge Management initiative, which seeks to provide a starting point for building true knowledge management (KM) capability in the pharma/biotech industry. This initiative, led by experts from across the industry, seeks to make the drug development journey better, cheaper and faster through improved quality, cost, speed, and agility.

A suggested hierarchy of knowledge management presents a familiar theme focusing on technology, process, and people. This hierarchy has, as its foundation, the proper IT infrastructure to adequately capture the information (content) generated by an organization during its development activities throughout the product life cycle. Ideally, the combination of IT infrastructure with centralized information will enable business processes based on best practices. These layers then come together to enable people to work smarter and faster, fostering agility, creativity, and leadership. Of course, this is easier said than done because just building databases on top of servers to aggregate information is not enough. The solutions that capture the information need to organize and contextualize the information to support key business processes and enable best practices. The fulfillment of these additional requirements is key to the conversion of information into institutional, actionable knowledge. This construct is a bit general, but it can be further specified starting with the deployment of novel SaaS applications to capture structured content enabling QbD/ICH workflows that drive shared process understanding.

It could be further argued that the establishment of a knowledge-based culture is not a linear hierarchy or a sequential set of activities (i.e. IT → Information → Process → People). Instead, each layer is cultivated in parallel with the others, starting with a small core of people (Agile teams) utilizing technology to enable more efficient processes, and then growing as Agile principles and more teams proliferate within the organization.

Is it Data, Information, or Knowledge?
The process development, manufacturing, and validation stream of activities for a new drug product average about 7-8 years in total cycle time. The total product life cycle can last over 20 years. Over that time, vast amounts of data are generated about the formulation, manufacturing process, recipes, materials, equipment, and more. Where does all this data end up?
For pre-commercial activities (PD/PV), it gets dispersed primarily in spreadsheets, reports, emails, and batch records which are converted into controlled documents and then stored in electronic quality management systems (eQMS). The process of aggregating the data into descriptive reports provides much-needed context transforming the content into more insightful information.
However, a fundamental problem still remains. At the end of a development cycle, an organization will have thousands of pages of information and data stored as unstructured narratives in various locations. When it is time to draft regulatory submissions or work with auditors and inspectors, a significant amount of personnel time is required to manually extract the information needed (if it can even be found) and provide context around it. The challenge becomes even more acute when it is time to complete the highly complicated process of technology transfer.

Aggregating unstructured data begins the process of converting data into information. But, often, the sheer volume of this information blocks the transition from information to knowledge.
This is a ubiquitous problem that is not specific to the pharma/biotech industry. An entire industry has been created to help companies mine and aggregate their vast amounts of data. However, we see this as complexity layered on top of complexity, and it keeps this tradition of dispersed technology solutions in place. What if we go the other way and create a structured environment where information can be put where it belongs, with the appropriate context as it is generated? Are there specific advantages conferred by this approach? Indeed there are the following with structured data approaches:
- provides context to content, turning data into information
- increase visibility and transparency by improving accessibility
- create reusable artifacts making knowledge institutional.
Does the specific structure of information matter?
Throughout the pharma/biotech industry today, the fundamental unit of information is “the document.” This is akin to saying that a sand grain is the fundamental unit of matter. We all know that documents have a lot of smaller increments of information, much like the sand grain is composed of many atoms and other subatomic particles.

Continuing with this analogy, what if we define the requirement as the atomic unit of information. Requirements are then defined, assembled, and linked to form a web of information. These can be patient requirements, product requirements, and process requirements with related information such as risk, acceptance criteria, manufacturing data, and so on, all linked together. This approach creates a multi-dimensional data set where each element represents a node that can be linked to other nodes.
This multi-dimensional data set can be probed in any subset of dimensions to understand the critical relationships within the subset of interest. Now we can begin converting information into knowledge.
Driving Best Practices
The structured, requirements-based approach to knowledge management presents significant opportunities to vertically integrate information in ways not previously possible with a document-based system.
Stage 1 of this framework starts with the requirements-based structure where patient, product, and process requirements can all be defined and tracked individually.
Stage 2 of vertical integration brings the tools of quality risk management (QRM) as described in ICH Q9 and Q10, into the framework. With QRM integration, each requirement can be separately assessed for risk. Furthermore, process requirements can be digitally linked to product requirements, and product requirements can be linked back to patient safety/efficacy attributes, creating risk-based traceability as recommended throughout ICH Q8 – Q10.
Stage 3 integrates raw material and manufacturing data into the multidimensional data set. This data can be analyzed to identify trends and assess process capabilities. The integration of process data drives a key feedback loop enabling a consistent focus on those areas which need the greatest attention.
Stage 4 folds in the tangible assets of production, specifically raw materials, components, and equipment. Visibility into these dimensions is often limited and not easily accessible. By tracking these assets with respect to qualification and performance and how they link to suppliers and supplier risk, a comprehensive view of the entire production process comes into focus (ICH Q10).
Stage 5 uses all of the other stages to justify the control strategies identified for the validated process. With the strong, foundational knowledge base represented by Stages 1-4, it becomes easier to define a robust control strategy and defend that strategy going forward. This knowledge base also simplifies the impact assessment of changes to the process post-approval.

“To QbD or Not to QbD?”
It is a central question always considered during process development in pharma and biotech. Quality-by-Design (QbD) is a philosophy of product development that seeks to design quality into a product (as opposed to testing quality into a product) and is relevant when the product is being designed and the process to manufacture it is being developed. This approach moves to the Lean Manufacturing and Six Sigma methodologies for continuous improvement once the commercial manufacturing process is finalized and implemented.
The QbD approach is generally equated with Design of Experiments (DoE) and often seen only as a set of activities to characterize variable interaction and establish a design space in manufacturing processes. What is generally missed is the understanding that QbD is more holistic. It is a systematic methodology for designing quality into products that:
- Begins with predefined objectives
- Emphasizes product and process understanding and process control
- Is based on sound science and quality risk management
In fact, this very language is codified into the ICH guidelines (see ICH Q8(R2)) such that the methodology of QbD is enshrined in the international regulatory framework. So, the question posed at the beginning of this section is a false one. It isn’t a question of whether or not to QbD, the question is how to do QbD.
QbD in the more holistic sense is considered a best practice for the entire product and process development lifecycle. The challenge has always been to meet the three requirements of QbD listed above in the absence of a structured, comprehensive knowledge management solution. The introduction of vertically-integrated knowledge management addresses this challenge definitively. The end result is the natural alignment of the development process with a holistic QbD strategy and ICH guidelines all underpinned by growing digital capabilities and maturity.
Process Understanding and Intelligence

The call to action going forward is “Know Where. Know How. Know Why.” It starts with always knowing where your information is located, presumably in a centralized source -of truth. Next, utilizing the information in a coordinated and collaborative manner to understand how it all comes together to demonstrate process control and the management of residual risk.
Vertically integrated knowledge management enables the aggregation and centralization of information within a structured repository creating this single source of truth. With all information centralized, this framework brings consistency, standardization, and shared access which then adds context and enables collaboration with this contextualized information. Layering in decision support tools combined with context starts the journey to more robust understanding as your knowledge base grows. As teams explore and characterize the evolving process from early development to production and CPV, a deeper understanding and intelligence emerges which can then be used to demonstrate robust process control and accelerate technology transfer activities digitally from one organization or one site to another.

Getting Agile
Agile methodologies have been commonly discussed over the past couple of decades and continue to receive significant attention as a recognized approach for improving the speed of product development and final product quality with lower risks and overall costs. This approach has been successfully deployed in the development of complex technology-based products ultimately transforming entire organizations.
More recently, there has been an increasing call for the deployment of agile methodologies in pharma and biotech R&D as a way to increase productivity and generate better outcomes. The term, Agile, can mean many things depending on the application, and this spectrum of definitions has generated a variety of implementation philosophies. However, there are some consistent themes that we can identify which support the application of agile to pharma R&D. Agile methodologies generally seek to:
- Move the product development process closer to the customer
- Focus the process on outcomes not features
- Create small fit-for-purpose teams that can be flexible and move quickly
- Empower teams to operate autonomously and iterate rapidly
Who is the “customer” in this context? Is it the patient? The manufacturing contractor? The regulators? In many cases, it is all of the above.
Do these principles fit with the regulatory requirements of pharma/biotech product development? Requirements-based knowledge management coupled with QbD/ICH workflows does move the process closer to each of the customer groups identified. An example is the philosophy of QbD where you “start with the end in mind”. More specifically, the end is the patient and you start your product development journey with an understanding of the patient needs via the Target Product Profile (TPP). Therefore, your knowledge management journey should begin with the TPP.
From the perspective of drug product quality, outcomes are defined as the attributes of the product that affect patient safety and efficacy. Again, the QbD/ICH process describes the need to identify the drug product attributes and to develop a clear understanding of how each attribute affects patient safety and efficacy. These attributes are ranked by risk and those attributes exceeding a specific threshold for risk are deemed as critical. From there the development program focuses on the manufacturing process to ensure that the product attributes deemed as critical meet the required specifications to ensure patient safety and efficacy.

The last two common elements of the agile methodology are operational paradigms where small, fit-for-purpose teams are established that can move quickly and operate autonomously.
These operational paradigms are not discussed in the QbD or ICH guidelines, but they are certainly relevant to improving the quality of outcomes. One example is a possible “team of teams” configuration where each team owns specific responsibilities which converge to cover the full set of development activities.
The McKinsey discussion (cited reference) of these themes also notes the need for a strong backbone of core processes, capabilities, and technologies to support agile operational modalities. Digital solutions for knowledge management constitute a critical “core capability and technology” which sit at the center of the agile teams’ activities providing access to a shared and evolving knowledge base. These teams can then work autonomously but still benefit from the aggregated and contextualized information generated by their activities to further support their activities and those of other teams throughout the lifecycle.

The agile approach enabled by vertically-integrated knowledge management improves R&D efficiency and, consequently, overall productivity by saving development costs, reducing cycle times, and increasing the probability of technical success.
Even marginal reductions in costs can yield a significant return on investment. Perhaps most importantly, this approach promotes the implementation of industry-recognized best practices and facilitates agile product development even within a highly regulated environment advancing organizations toward the goal of operational excellence.
In future KML whitepapers, we will continue to explore in more detail how vertically-integrated knowledge management supports the agile development journey for pharma/biotech R&D.