The future unfolds at the 2025 Digital CMC Summit
February 25-27, 2025 • Austin, TX
Here’s why you should
watch the summit on-demand
Once again, this world-class event brought together the brightest minds in drug development to explore the cutting edge of technical development. This year’s focus: How digital and AI technologies are already reshaping programs across the industry.
Missed the live event? Catch up on seven in-depth discussions from the summit, now available on-demand!
It was a can’t-miss event for:
- Process Development
- Information Technology
- MSAT
- Analytical Development
- Innovation Leaders
- CMC Leads
- DS/DP Development
- Facilities Design
- Quality Assurance
- Validation Scientists
50+
100+
INDUSTRY LEADERS
20+
EXPERT-LED SESSIONS
TOP15
Pharma & Biotech Speakers
2025 Speakers & Panelists











Keynote Spotlight | Doug Hurley
Astronaut ★ U.S. Marine Colonel ★ Speaker ★ Trailblazer
Scaling Innovation in Highly Regulated Industries: Lessons from Space
See how inventive thinking, resilience, and a fail-forward mindset can deliver big results, even in complex regulatory frameworks.

Watch on-demand sessions
from the 2025 Summit
Get groundbreaking insights on drug development’s accelerating transformation, straight from the stage of 2025’s 3rd Annual Digital CMC Summit
Project Artemis: Building a Functioning and Sustainable Digital CMC Ecosystem
Discover how this new initiative aims to drive industry-wide collaboration, promote standardization & acceleration adoption of Digital CMC
Fostering a Digital Culture & Effective Change Management
Learn the keys to building a digital culture that breaks down resistance to change and sustains transformation at scale.
CMC Data: The New Frontier for BioPharma Gold Rush
In this year’s Lightning Talks, get rapid-fire insights on AI, automation, and the digital workflows redefining CMC efficiency.
Real World Use-Cases for CMC Digitization and Solutions
Hear from leading drug developers who are already implementing successful Digital CMC strategies that deliver tangible impact.
Connecting Digital CMC Ecosystem
Discover how next-gen strategies and technologies are helping CMC programs eliminate silos, transform decision-making, and enhance productivity.
The Future of
QbDVision
See what’s next for the world’s leading Digital CMC platform, and how it’s helping technical development users unlock the power of AI and automation
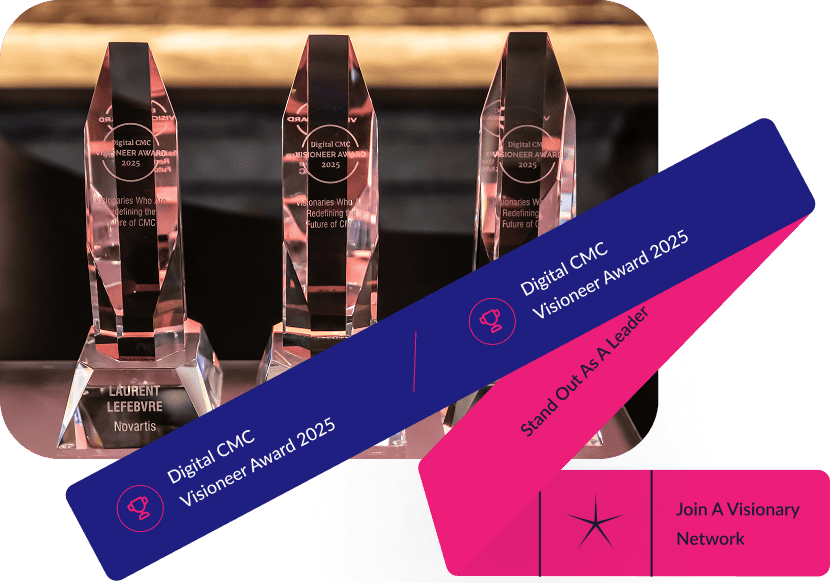
The Digital CMC
Visioneer Awards
At this year’s Summit, recognized the leaders whose clarity of vision, innovation, and dedication are shaping the future of drug development.
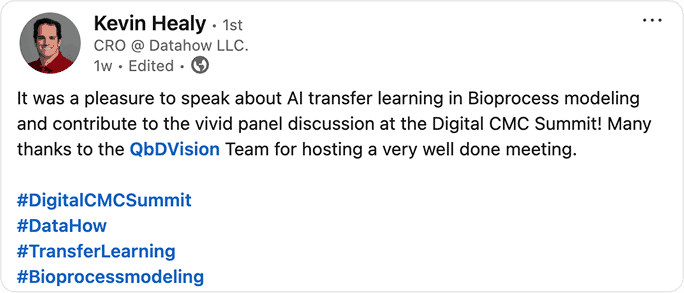
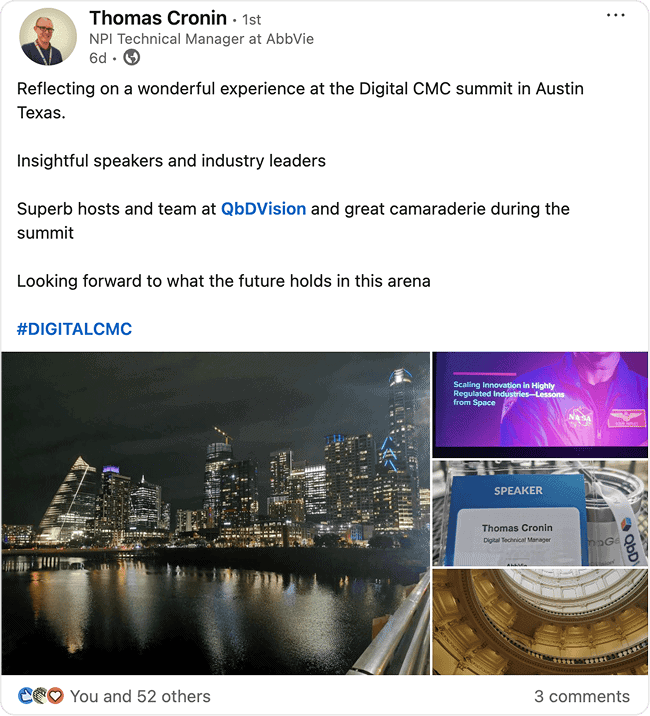
Attendee raves
See what attendees are saying about our event! Discover how our sessions and community impact their work, and why they’re excited to be part of this journey.


Innovation Challenge Turning Ideas into Action
At the Digital CMC Summit, we didn’t just talk about the future of pharma – we built it. Through our Innovation Challenge, cross-functional teams came together to tackle real industry bottlenecks, applying fresh thinking to areas like AI-driven automation, regulatory compliance, and knowledge transfer.
The result? Big ideas with real impact!
Catch up on the highlights and see the winning concepts
Who attended the Summit?
Our diverse attendees come from key sectors such as Pharma, Industry Groups, CDMOs, Biotech, Regulatory, and more…
Attendees
Seniority Levels
Industry
How to get involved in future events
Discover how you can actively contribute and engage with the Digital CMC community
2025 Summit Sponsors
We are proud to feature our Gold and Silver sponsors. Their support plays a vital role in making this event possible.
Interested in joining them as a sponsor? Click below to learn more and get involved!
Gold sponsors

Brought to you by the pioneers of CMC transformation
The Digital CMC Summit is proudly brought to you by QbDVision: developers of the industry’s first and only integrated Digital CMC management platform.


Stay up-to-date on Digital CMC events
If you found the Digital CMC content engaging, we invite you to join us at our in-person summit. Stay updated on the latest announcements regarding Digital CMC events & content by signing up below.




























































