Read more from Yash
- All Posts
All Posts
- All Posts
Collaborating on Quality Risk Management for New Drugs at IFPAC 2021
Yash Sabharwal
February 23, 2021
IFPAC 2020 was the last in-person conference we attended before the COVID-19 pandemic reshaped our industry’s major global conferences. We're excited to be back!
Read more
Risk Ranking for Product Quality Attributes
Yash Sabharwal
February 9, 2021
Since its inception, QbDVision has integrated risk assessment functionality into its core. Explore our platform's latest risk ranking functionality.
Read more
Yield Optimization or Product Quality? Yes.
Yash Sabharwal
January 28, 2021
Companies developing and manufacturing biologics are heavily focused on product quality metrics to ensure patient safety and efficacy. They are equally focused on the optimization ...
Read more
Track Process Evolution with Digital Process Management
Yash Sabharwal
January 25, 2021
As your team cycles through the development process, there is one constant: change underpinned by learning from each phase. Here's the digital way to manage ...
Read more
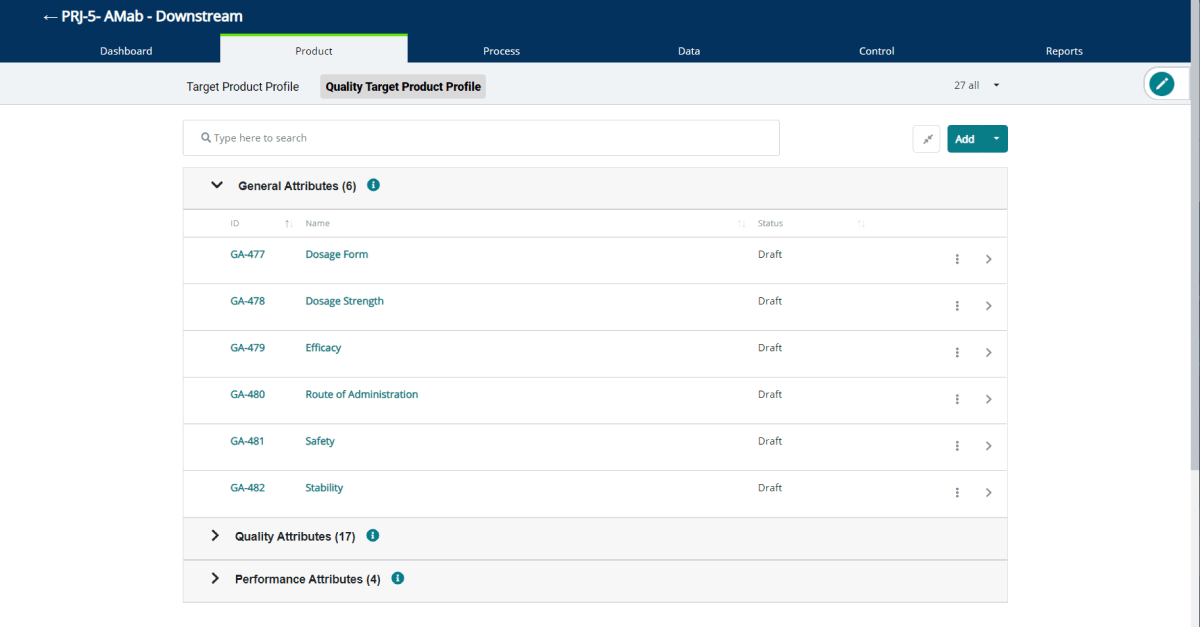
Introducing General Attributes: The Hidden Structure to the QTPP
Yash Sabharwal
December 15, 2020
We’ve added a new record type called a General Attribute (GAs) as part of the definition of the Quality Target Product Profile (QTPP). Explore this ...
Read more
Digitally Managing the Evolution of Your QTPP
Yash Sabharwal
December 14, 2020
The Quality Target Product Profile or QTPP is a prospective summary of the quality characteristics of a drug product that ideally will be achieved to ...
Read more
First Time Right: The Key to Effective Product & Process Development
Yash Sabharwal
November 5, 2020
Our industry faces numerous challenges related to scaling manufacturing processes for cell and gene therapies. Explore some key recommendations and potential solutions.
Read more
CherryCircle Software Raises $2MM in Additional Seed Financing
Yash Sabharwal
October 15, 2020
Yes indeed, CherryCircle just knocked out an additional round of seed financing. Combined with our last raise, brings our total fundraising to around $4.6M.
Read more
FDA Releases New Guidance on Manufacturing Operations
Yash Sabharwal
September 11, 2020
Today, the FDA issued temporary guidance on the resumption of normal manufacturing operations during the COVID-19 health emergency.
Read more
Harness your expertise
See how QbDVision can help you leverage your team’s know-how to accelerate drug development.